Περιγραφή:
Methylenedioxymetampetamine (MDMA), known initially with the brand name “Ecstasy” was originally developed in 1912 and was later promoted as a treatment for narcolepsy in selected patients. The drug was banned in 1985 due to safety concerns. In the late 1980s “Ecstasy” became an embracive term of pills that can contain a mixture of diverse substances including MDMA, such as LSD, cocaine, amphetamines, caffeine, heroine, rat poison, antiparasites etc. This is what makes “ecstasy” particularly dangerous since the user never really knows what combinations he is taking.
A 17 year old male presented to the Emergency Department of our hospital after ingesting one tablet of a substance, which he believed was a synthetic “ecstasy” combined with alchohol. He and his friends reportedly ingested the same substance at a party. He had no prior health problems and received no medication. After the initial euphoric effects subsided, he experienced lightheadedness, nausea, vomiting and diaphoresis. On arrival to the Emergency Department he had a decreased level of consciousness, hyperpyrexia (39,8 ̊C) and marked hypoglycemia (2 mg/dl). He was haemodynamically unstable with hypotension (BP 80/60 mmHg) and developed cardiogenic shock. He was immediately intubated and put under mechanical ventilatory support. Patient’s blood and urine samples were collected at the time of admission before any medication were administered for drug testing.
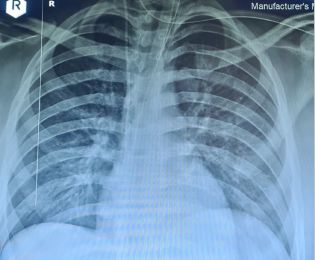
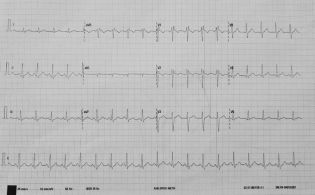
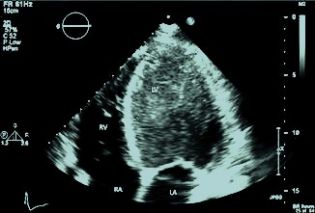
Electrocardiogram revealed sinus tachycardia ~130/min. Laboratory results reached a maximum value of white blood cells up to 44,5 K/μl, hematocrit 21,9 % platelets 92,2 K/μl, INR 2,06, D-dimers 4283 μg/l, SGOT 160 U/L SGPT 327 U/L, total bilirubin 28 mg/dl, CPK 1207 U/L, CRP 18,9 mg/L. Troponin I peaked at 5680 pg/ml. Chest X ray exhibited a normal cardiothoracic ratio with mild signs of pulmonary congestion. Echocardiography obtained at the day of admission revealed mild left ventricular dilatation with a severely impaired overall systolic function (EF~35%) and diffuse hypokinesis. The patient was transferred to the intensive care unit due to the developing polyorganic failure. He was put under inotropic and vasoconstrictory support. The patient developed rhabdomyolysis and acute renal failure that required continuous peritoneal dialysis. The patient also developed acute ischemic hepatitis with thrombocytopenia and transaminasemia with values 5 times above normal and total bilirubin up to 31 mg/dl that required plasmapheresis and fresh frozen plasma and platelet transfusions and prothrombin complex administration. Systolic dysfunction progressively improved and the patient was stabilized with an estimated ejection fraction approximately 55% three days later. They patient did no longer require pharmacological circulatory support.
Testing of toxicant exposure revealed the presence of 18 different substances, with predominant MDMA, MDA and benzoylecgonine. The pill included diverse substances including amphetamines, barbiturates, benzodiazepines, b blocker, paracetamol and caffeine (table 1). The patient is still hospitalized after hepatic transplantation. Cardiac and renal function have fully resumed, and the patient is heamodynamicallly stable.
Συμπέρασμα: Studies patients who received amphetamine derivatives at lowdoses for ADHD described a wide range of cardiac adverse effects, which did not include cardiomyopathy. This case demonstrates that street drug names can be misleading as the actual contents of the pills may be unknown both to the user as well as the clinician. In vitro human studies show that the metabolism of most psychostimulants is regulated by the cytochrome P450. This may explain individual differences in intensity of drug effects, susceptibility to acute toxicity and drug interactions. In our case a possible enzyme mutation and insufficiency could explain the severity of side effects experienced by our patient compared to his friends who demonstrated no toxicity after ingestion of the same pills.
Φαρμακευτική αγωγή:
Ing Dobutamine IV
Ing Dopamine IV
amp Furosemide IV
Εξέχουσες Δημοσιεύσεις
Γνώμες Ειδικών

Καρδιαγγειακά νοσήματα και σύγχρονος τρόπος ζωής
Κωνσταντίνος Τούτουζας

Συμμετοχή σε άρθρο για την Οπτική Συνεκτική Τομογραφία
Κωνσταντίνος Τούτουζας

Πόνος στο στήθος: Τι πρέπει να κάνετε;
Αγγελόπουλος Παναγιώτης

Μελέτες Επεμβατικής Καρδιολογίας το 2021: Χαμένοι και Κερδισμένοι
Κωνσταντίνος Τούτουζας

Σταχυολογήματα από το TCT 2021
Κωνσταντίνος Τούτουζας

Βασικά σημεία των νέων κατευθυντήριων οδηγιών για τις βαλβιδοπάθειες
Κωνσταντίνος Τούτουζας

Νέες Κατευθυντήριες οδηγίες για τις Βαλβιδοπάθειες
Κωνσταντίνος Τούτουζας

Πρακτικές στο Αιμοδυναμικό Εργαστήριο
Κωνσταντίνος Τούτουζας

FDA Clears Abbott's AI-Powered Coronary Imaging Platform
Κωνσταντίνος Τούτουζας

Aντιθρομβωτική θεραπεία σε προσθετικές καρδιακές βαλβίδες
Μ.Μπότης,Γρ.Παττακός,Ι.Γουδέβενος

Οι ανεμβολίαστοι (και το μυαλό τους) παραμένουν το πρόβλημα
Κοχιαδάκης Γεώργιος

Καρδιά και καύσωνας: Μέτρα προστασίας
Αγγελόπουλος Παναγιώτης

Αντιμετωπίζοντας τις παράπλευρες απώλειες του κορωνοϊού
Λάμπρος Μιχάλης

Πώς ξεχωρίζουμε απλές παρενέργειες εμβολίων από τις καρδιακές επιπλοκές
Κωνσταντίνος Τούτουζας

Κρίστιαν Έρικσεν
Κωνσταντίνος Τούτουζας

Στατιστικά Μαϊου για την Covid-19
Κοχιαδάκης Γεώργιος

Η καρδιά είναι φτιαγμένη να χτυπά
Κοχιαδάκης Γεώργιος

Άρση των πατεντών για τα εμβόλια. Προσδοκίες και ευκαιρίες
Κωνσταντίνος Τούτουζας

Οι θέσεις των Αμερικανικών Ιατρικών Ενώσεων για τη θρόμβωση μετά τον εμβολιασμό
Κοχιαδάκης Γεώργιος

COVID-19: τι μάθαμε σ’ ένα χρόνο
Τρίκας Αθανάσιος

Υποχρεωτικός και αναγκαστικός εμβολιασμός – Η σύγχυση προκαλεί αντιδράσεις
Σουλιώτης Κυριάκος

Οι παράπλευρες συνέπειες της COVID-19
Μιχάλης Λάμπρος

4 Λόγοι γιατι η τακτική άσκηση είναι σημαντική για τους καρδιακούς ασθενείς
Αγγελόπουλος Παναγιώτης

Η επίδραση της πανδημίας COVID-19
Παπαφακλής Μιχαήλ

Το FDA εγκρίνει το WATCHMAN FLX
Γεώργιος Τρανταλής,
Κωνσταντίνος Τούτουζας

Μελέτη ISCHEMIA: Τι αλλάζει στην κλινική πράξη;
Κωνσταντίνος Τούτουζας

Αιμοδυναμικό Εργαστηρίο & COVID-19
Λάμπρος Μιχάλης

Κορονοϊός και Καρδιά
Κωνσταντίνος Τούτουζας






Η αξιολόγηση από ομότιμους είναι ευθύνη της Cardio Act
Σύγκρουση συμφερόντων: Δεν δηλώθηκε
© 2019 CardioAct